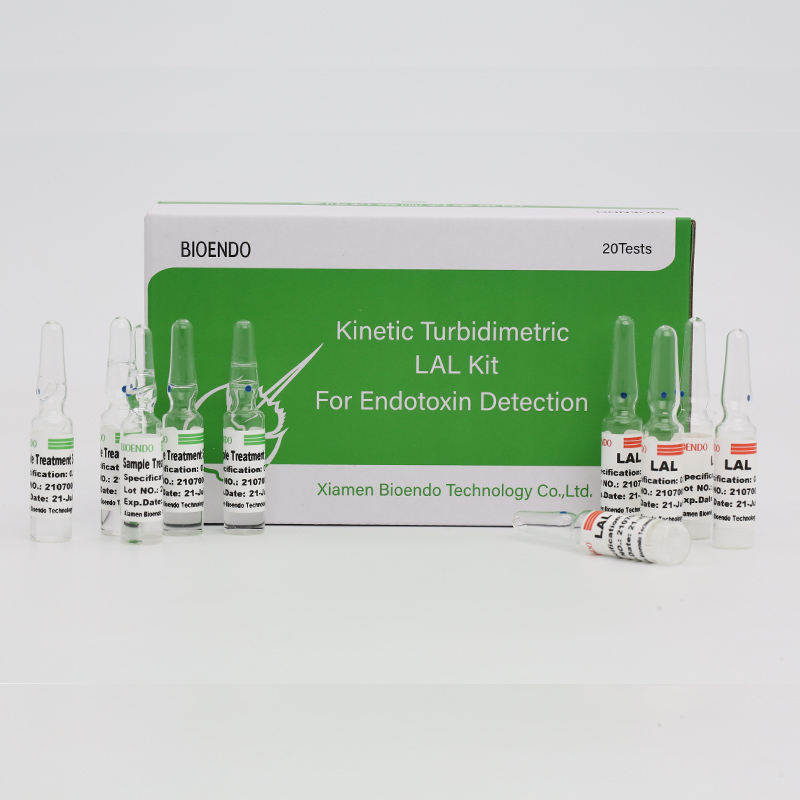
ʻO Endotoxin Assay Kit for Human Plasma
ʻO Endotoxin Assay Kitno ka Plasma Kanaka
1. ʻIke huahana
Hoʻopau ʻia ka CFDAʻO ka pahu hōʻike hōʻailona Endotoxinka helu endotoxin pae inhuman plasma.ʻO Endotoxin kahi mea nui o ka paia o ke kino o Gram Negative bacteria a ʻo ia ka mea koʻikoʻi microbial mediator o sepsis.Hiki i nā kiʻekiʻe kiʻekiʻe o ka endotoxin ke hoʻoulu pinepine i ke kuni, hoʻololi i ka helu keʻokeʻo keʻokeʻo a, i kekahi mau hihia, haʻalulu puʻuwai.Hoʻokumu ʻia ia ma ka helu Cpathway ma ka limulus Polyphemus (ke koko pāpaʻi lio).Me ka mea heluhelu microplate kinetic a me ka polokalamu ho'āʻo endotoxin, ʻike ʻo Endotoxin assay kit i ka pae endotoxin i loko o ka plasma kanaka ma lalo o hoʻokahi hola.Hele mai ka kit me ka plasma pre-treatment reagent e hoʻopau i nā mea pale i ka plasma i ka wā o ka endotoxin assay.
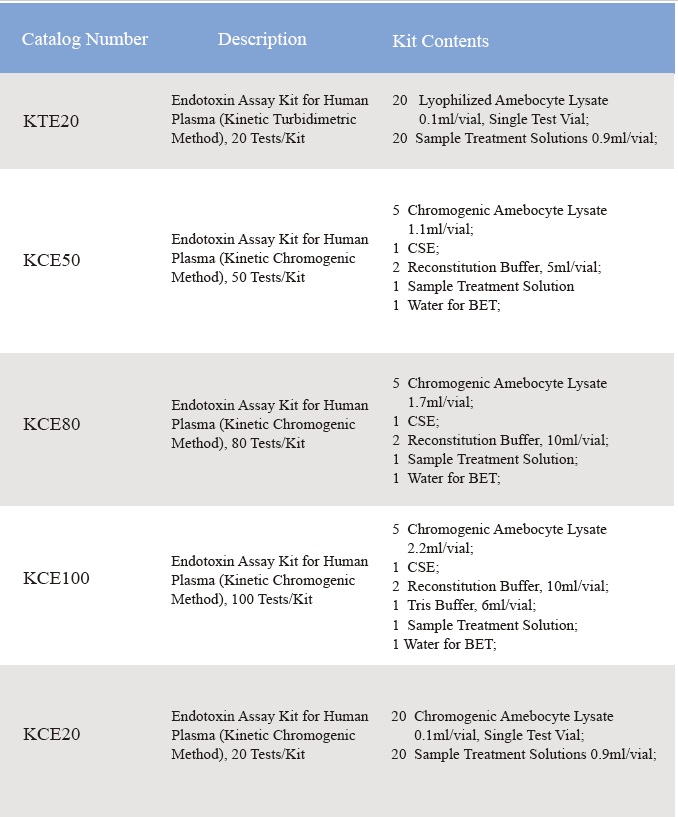
2. Lahui Huahana
Laulā ho'āʻo: 0.01-10 EU/ml
3. Hiʻona Huahana a me ka noi
Hele mai me ka plasma pretreatment solutions, e hoopau i na mea inhibition i ka plasma kanaka.
Nānā:
ʻO Lyophilized Amebocyte Lysate (LAL) reagent i hana ʻia e Bioendo ua hana ʻia mai ka amebocyte lysate i loaʻa i ke koko o ka pāpaʻi lio.
Ua ʻike ʻia ka naʻau o Lyophilized Amebocyte Lysate a me ka mana o Control Standard Endotoxin e kūʻē i ka USP Reference Standard Endotoxin.E hele mai ana nā kit reagent Lyophilized Amebocyte Lysate me ke aʻo huahana, Certificate of Analysis, MSDS.