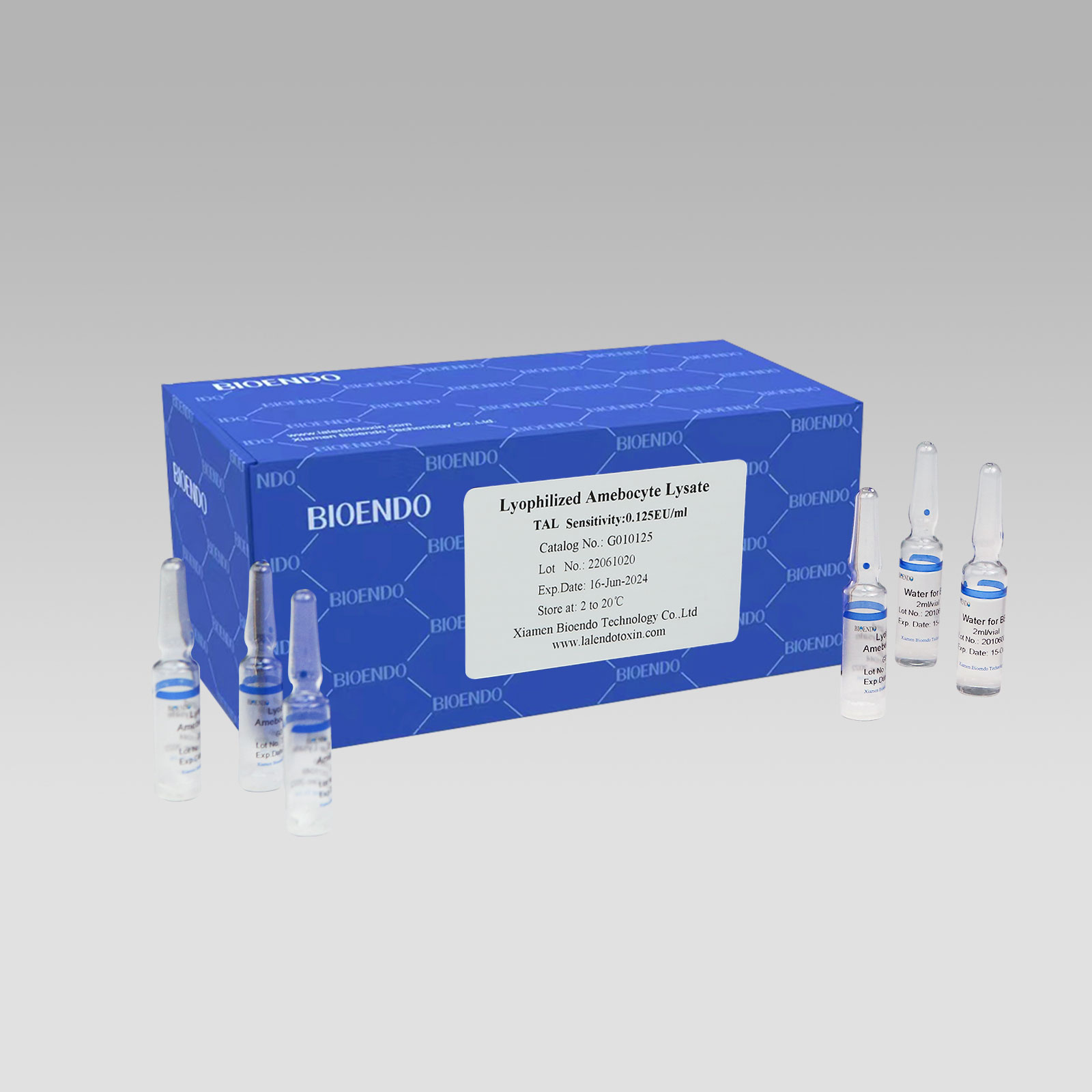
Gel Clot Lyophilized Amebocyte Lysate Ho'āʻo hoʻokahi ma Ampoule G01
ʻO Gel Clot Lyophilized Amebocyte Lysate pahu hōʻike hoʻokahi (Glass Ampoule G01)
ʻO ka Gel Clot Lyophilized Amebocyte Lysate (LAL) Single Test ma Ampoule he ʻano o ka endotoxin test assay i hoʻohana nui ʻia i nā ʻoihana lāʻau lapaʻau a me nā mea lapaʻau e ʻike ai i ka loaʻa ʻana o nā endotoxins i nā laʻana like ʻole.
Eia kekahi mau hiʻohiʻona o kēia hōʻike hoʻāʻo endotoxin hoʻokahi:
1. Hoʻonaʻauao: ʻO ka Gel Clot Single LAL Test ma Ampoule he paʻakikī loa a hiki ke ʻike i nā pae endotoxin ma lalo o 0.03 EU / mL.
2. Mea kiko'ī: Ho'āʻo endotoxin kikoʻī, kikoʻī loa ka hoʻāʻo ʻana i nā endotoxins a ʻaʻole hoʻololi i ke ʻano me nā mea ʻē aʻe o ka hāpana.
3. Hoʻoluʻolu: ʻO ke ʻano hōʻike hoʻokahi o ka Gel Clot Single LAL Test ma Ampoule e maʻalahi a maʻalahi hoʻi e hoʻohana, no ka mea e hoʻopau i ka pono no ka hoʻomākaukau ʻana i nā reagents a me nā pihi maʻamau.
4. Paʻa: ʻO ke ʻano lyophilized o nā reagents LAL e hāʻawi i ka paʻa maikaʻi loa, e ʻae i ka hoʻāʻo e mālama ʻia ma ka lumi wela no nā manawa lōʻihi me ka ʻole o ka hoʻokō ʻana i kāna hana.
5. ʻO ke kumu kūʻai: ʻO ke ʻano hoʻāʻo hoʻokahi o ka Gel Clot LAL Single Test ma Ampoule ʻoi aku ka maikaʻi ma mua o nā ʻano like ʻole o ka endotoxin test assays, e like me ka kinetic chromogenic LAL assay, kinetic turbidimetric LAL assay, end-point chromogenic LAL hōʻike hoʻāʻo a me recombinant factor C endotoxin test assay.
ʻO ka Gel Clot Single LAL Test ma Ampoule he ala hilinaʻi, maʻalahi, a maʻalahi hoʻi no ka ʻike ʻana i nā endotoxins i kahi ākea o nā laʻana, e lilo ia i mea pono no ka hōʻoia ʻana i ka palekana a me ka maikaʻi o nā huahana lāʻau lapaʻau a me nā lāʻau lapaʻau.
1. ʻIke huahana
Gel Clot Lyophilized Amebocyte Lysate ho'āʻo hoʻokahii loko o ka ampoule Loaʻa i ka endotoxin-specific Amebocyte Lysate e komo pū ana me ka beta-glucan inhibitor i loko o ka hoʻokumu ʻana a ʻaʻole e pane i ka beta-glucan.No kā mākou hoʻāʻo hoʻokahi i ka ampoule aniani, hiki iā ʻoe ke hoʻohui pololei i ka laʻana i nā ampoules aniani.ʻAʻole pono ʻoe e hana hou i ka Amebocyte Lysate ma mua, a e hoʻoholo ʻoe i ka nui o nā hoʻāʻo e hoʻohana i kēlā me kēia manawa e pale aku i ka ʻōpala, ʻo ia hoʻi ka ʻokoʻa nui mai ka endotoxin testing multi test vial.Pono nā paipu manuahi Endotoxin no ka dilution o Lyophilized CSE.ʻO ka hana o ka ʻike endotoxin e hoʻohana i ka Bioendo Gel Clot Lyophilized Amebocyte Lysate Single Test i ka ampoule e like me ka Pharmacopoeia aupuni.
2. Lahui Huahana
ʻO ka hoʻāʻo ʻana o ka gel clot assay single test glass ampoule.
Nā Manaʻo: 0.03EU/ml, 0.06EU/ml, 0.125EU/ml, 0.25 EU/ml, 0.5EU/ml.
3.Product Hiʻona a me ka noi
Hoʻokahi ʻanuʻu endotoxin ʻike, maʻalahi e hoʻohana i ka hoʻokolohua o ka hoʻāʻo endotoxin bacteria.
Loaʻa ka ʻauʻau wai maʻamau a i ʻole ka incubator wela maloʻo no ka hoʻoulu ʻana o ka gel clot method LAL reagent.
He kūpono nohoʻāʻo endotoxin huahana hopema mua o ka hoʻokuʻu ʻia ʻana o ka huahana.
E hana i ka ho'āʻo hoʻāʻo endotoxin, ua hoʻopaʻa ʻia ka naʻau kits e like me ka China Pharmacopoeia criterion.
Nānā:Hana ʻia ka Lyophilized Amebocyte Lysate (LAL) i hana ʻia e Bioendo mai ka lysate o nā amebocytes (keʻokeʻo koko keʻokeʻo) mai ka pāpaʻi kāmaʻa lio.
| Heluhelu No. | Hoʻonaʻauao EU/ml | wehewehe | Kiko kiko |
| G010030 | 0.03 | ʻO Bioendo Gel Clot Endotoxin Test Kit, Ho'āʻo hoʻokahi ma Ampoule. | 50 mau ho'āʻo no ka pūʻolo |
| G010060 | 0.06 | ||
| G010125 | 0.125 | ||
| G010250 | 0.25 | ||
| G010500 | 0.5 |
Ua ʻike ʻia ka naʻau o Lyophilized Amebocyte Lysate a me ka mana o Control Standard Endotoxin e kūʻē i ka USP Reference Standard Endotoxin.Hele mai ka Lyophilized Amebocyte Lysate reagent kits me ke aʻo huahana, Certificate of Analysis.
No ke aha i koho ʻia ai ka pahu hoʻāʻo gel clot G01:
1. ʻO ka mea ho'āʻo maikaʻi loa a hoʻohana pinepine ʻia no ka ʻike endotoxin.
2. ʻO ka hoʻāʻo hoʻokahi i loko o kahi ampoule no hoʻokahi ʻanuʻu e hōʻemi i ka pilikia o ka contamination.
3. Gel clot assay hoʻokahi hōʻike aniani ampoule ʻaʻole pono ka mea heluhelu microplate akamai.
4. E ho'āʻo i nā endotoxins ma ke kaʻina hana o ka endotoxin test assay.
Nā huahana pili i ka endotoxin test assay:
ʻO ka wai no ka hoʻāʻo ʻana i ka Endotoxins Bacterial (BET), Manaʻo iā TRW02, TRW50 a i ʻole TRW100,
Endotoxin free glass tube (dilution tube), Manaʻo T1310018 Pyrogen nā ʻōlelo aʻoaʻo manuahi, Manaʻo PT25096 a i ʻole PT100096
Pipettor,Manaʻo iā PSB0220
ʻO ka pahu hoʻāʻo,
Mea Hana Hoʻopā (Water Bath or Dry Heat Incubator ),
Manaʻo ʻia i ka Incubator Heat Dry TAL-M2
Vortex Mixter, Manaʻo VXH.
Manaʻo Endotoxin maʻamau, CSE10A.